 In life, radiation is integrated into three different types, Alpha, beta and Gamma Radiation. You must have all heard of the term 'Radiation', but this post is more concerned about Radiation into specifics. But not too complicated. Just a basic idea of the types of radiation and the hazards each radiation poses. After all, not all of you want to be Radiologists. BUT we do want to be able to save our selves from the adverse affects of technology, hence emphasizing the importance of understanding all this.
In life, radiation is integrated into three different types, Alpha, beta and Gamma Radiation. You must have all heard of the term 'Radiation', but this post is more concerned about Radiation into specifics. But not too complicated. Just a basic idea of the types of radiation and the hazards each radiation poses. After all, not all of you want to be Radiologists. BUT we do want to be able to save our selves from the adverse affects of technology, hence emphasizing the importance of understanding all this.Before we continue, I want to loosely define the term radiation: Wikipedia defines radiation as: radiation is a process in which energetic particles or energy or waves travel through a medium or space
Now we got that out of the way, lets move on into what we really want to know
Alpha Radiation
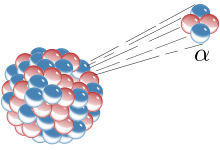
The picture on the right is an Alpha radiation model. The blue and red bits symbolize Neutrons and Protons. I will do a separate post about that later on.
Alpha Radiation is a type of 'energy' decay which releases an Alpha particle. This transforms into into an atom with a mass number 4 less and atomic number 2 less, also equivalent to that of a Helium Atom.
The example below is a good illustration of what I'm talking about.
| 238 92U | → | 234 90Th | + | 4 2He2+ |
The Uranium loses 4 mass numbers from 238 to 234 and it also loses two protons; from 92 to 90. If you don't understand what I'm talking about, feel free to check out my tutorials on Protons, Neutrons and Electrons.
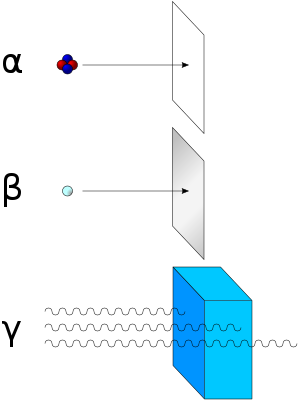
Alpha Particles have very little energy. Alternatively, we can say that it has a low penetrating power.. If you zap a ray of Alpha Particles into a sheet of paper, most of the particles will be absorbed. That's how weak it is.
Beta Particles
Beta is the particle in the middle. Beta radiation does not release a Helium Atom, but a Electron, hence making it negatively charged. Take a look at this example.

A neutron is converted into a Proton, and in the process, an electron and an electron anti neutrino is emitted. Lets forget about the electron anti-neutrino, that is hardly elementary, and just remember an electron is emitted, and that beta is NEGATIVELY charged. ok, good.
The 'Penetrating' Power of Beta Radiation is much stronger than that of Alpha, as it can easily penetrate paper. However, it cannot penetrate a piece of Aluminium, which is of an order of magnitude thicker than that of paper!
Gamma Radiation
I am pretty sure most of you have heard of this type of radiation.
Thankfully, Gamma Radiation carries no charge. However, it carries the most penetrating power, being able to comfortably penetrate most of a thick piece of lead. It is a form of electromagnetic radiation which can cause genetic mutations
There is a whole lot more about Gamma radiation, for this lesson, i'll adhere to brevity and simplicity.
No comments:
Post a Comment